Understanding what happens to phosphate fertiliser when it is applied to soil is an advantage when developing fertiliser recommendations, especially if reductions in fertiliser use are being considered.
Phosphorus is a relatively immobile nutrient and ultimately, most of the phosphorus applied in fertiliser is potentially available for plant uptake. However, the extent and dynamics of this will vary, based on several factors, including:
- The solubility of the phosphate applied
- The soil’s ability to retain phosphate
- Soil pH
- The degree to which applied phosphate is used by soil micro-organisms
Why apply phosphate fertiliser?
An adequate supply of phosphorus is essential for plant growth, and is especially important during germination, root growth, tillering, seed and fruit setting, and ripening. Most New Zealand soils are naturally deficient in phosphorus, so fertiliser application is needed to support high-producing pastoral farms, arable crops and horticultural operations.
Fate of soluble phosphate
Most phosphate fertiliser applied to New Zealand farms contains soluble phosphate, e.g. as found in single superphosphate, diammonium phosphate (DAP) and triple superphosphate. After application there is a rapid rise in the concentration of phosphate in the soil water. This phosphate has several possible fates:
- It may be taken up by plants
- It may be used by soil micro-organisms (i.e. immobilised as organic matter)
- It may adsorb on to positively charged sites on soil particles
- It may react with other minerals in the soil
Phosphate adsorption
Soil particles have various minerals on their surfaces, including iron and aluminium oxides and alumina silicates. These particular minerals are positively charged, and since phosphate is negatively charged, it will adsorb to these sites in an electrostatic and chemical interaction (see Figure 1). This is reversable – as the concentration of phosphate in the soil falls (for example, as plants use it), adsorbed phosphate will be released to replenish reserves.
Over time, this adsorbed phosphate can become less able to desorb. This happens if the phosphate ions migrate below the surface of the soil – the phosphate is then absorbed. Similarly, adsorbed phosphate can become occluded, which also prevents it from desorbing. Occlusion occurs when the adsorbed phosphate is covered by an aluminium or iron oxide coating.
Phosphate precipitation
Soluble phosphate can also react with minerals in soil to form insoluble compounds. If the soil pH is less than 5.0, phosphate reacts with iron and aluminium, forming iron and aluminium phosphates. If the soil pH is greater than 6.0, phosphate reacts with calcium to form insoluble calcium phosphate. If dicalcium phosphate is formed, then over time the phosphate will be released, as it is moderately soluble. However, if tricalcium phosphate forms, then the phosphate is unlikely to be released naturally, as this compound is very insoluble. So maintaining pH near 6.0 is important to keep soil phosphorus available.
Effect of anion storage capacity
Anion storage capacity (ASC) is a measure of the ability of a soil to hold or bind negatively charged ions, such as phosphate. The ASC depends in large part on the soil’s origin; for example, soils of volcanic origin (e.g. allophanic, pumice and granular soils) have a relatively high ASC (70-90%) as a result of their naturally greater levels of phosphate-adsorbing colloids. In contrast, soils of sedimentary origin have lower levels of these colloids, and a correspondingly lower ASC (20-60%).
The ASC is a relative index, so an allophanic soil with an ASC of 90% will bind three times as much phosphate as a pallic soil with an ASC of 30%.
The ASC dictates how much phosphorus is required to raise the Olsen P of a soil by one unit (see Figure 2). The higher the ASC, the more sites there are to fill with phosphate, so the more fertiliser is required to fill those sites. This is why an allophanic (ash) soil requires 11 kg P/ha to raise the Olsen P by one unit, while a sedimentary soil only requires 5 kg P/ha to achieve the same result.
The ASC of a soil also influences what happens when phosphate fertiliser is withheld. The capital phosphate fertiliser added to the soil to raise the Olsen P builds up a reserve in the soil, and this is slowly released and used by plants. A study on soils with a high ASC and an Olsen P of 16, 34 or 60, found that withholding phosphate fertiliser had no effect on pasture production for at least five years (Roberts & Thomson, 1987).
In contrast, when phosphate fertiliser is withheld from a sedimentary soil, pasture production declines immediately. The lower ASC of this soil type means there is a much smaller pool of phosphate in the soil, so reserves are quickly depleted.
Effect of soil pH
Soil pH influences the adsorption of phosphate to soil minerals because it affects the amount of surface charge on these and other soil substances. As soil pH falls (i.e. soil becomes more acidic) there are more positive charges on the soil colloids, so more phosphate can be adsorbed. In principle, raising the soil pH will increase the proportion of negative charges on soil colloids, so releasing phosphate into soil water.
In practice, no pasture production response to lime application is seen above pH 5.8 (which is one reason why the recommended pH range for pastoral soils is 5.8-6.0).
If soil pH is going to be raised to increase phosphorus availability, then economics must also be considered. On country where lime needs to be applied by aerial topdressing, there is no economic response if soil pH is above 5.5.
A study of 25 trials examined the impact of lime on pasture production after phosphate fertiliser application to soils with a pH in the range 5.0 to 6.0 (Mansell et al., 1984). Of these 25 sites, only four had an economic pasture production response to the application of phosphate and lime, compared to the application of phosphate fertiliser or lime alone.
The application of lime to ameliorate soil acidity has clear benefits, e.g. reduction of aluminium toxicity, increasing molybdenum availability and increasing the rate of nitrogen mineralisation. However, its use purely to increase phosphate availability is not likely to be warranted.
Download and read the full fact sheet

Understanding phosphorus on your farm
Most New Zealand soils are naturally deficient in phosphorus (P). On farmland, high production of animal and horticultural products exacerbates deficiencies if the phosphorus removed is not replaced. Therefore, the use of phosphorusbased fertilisers is vital for the success of New Zealand agriculture.
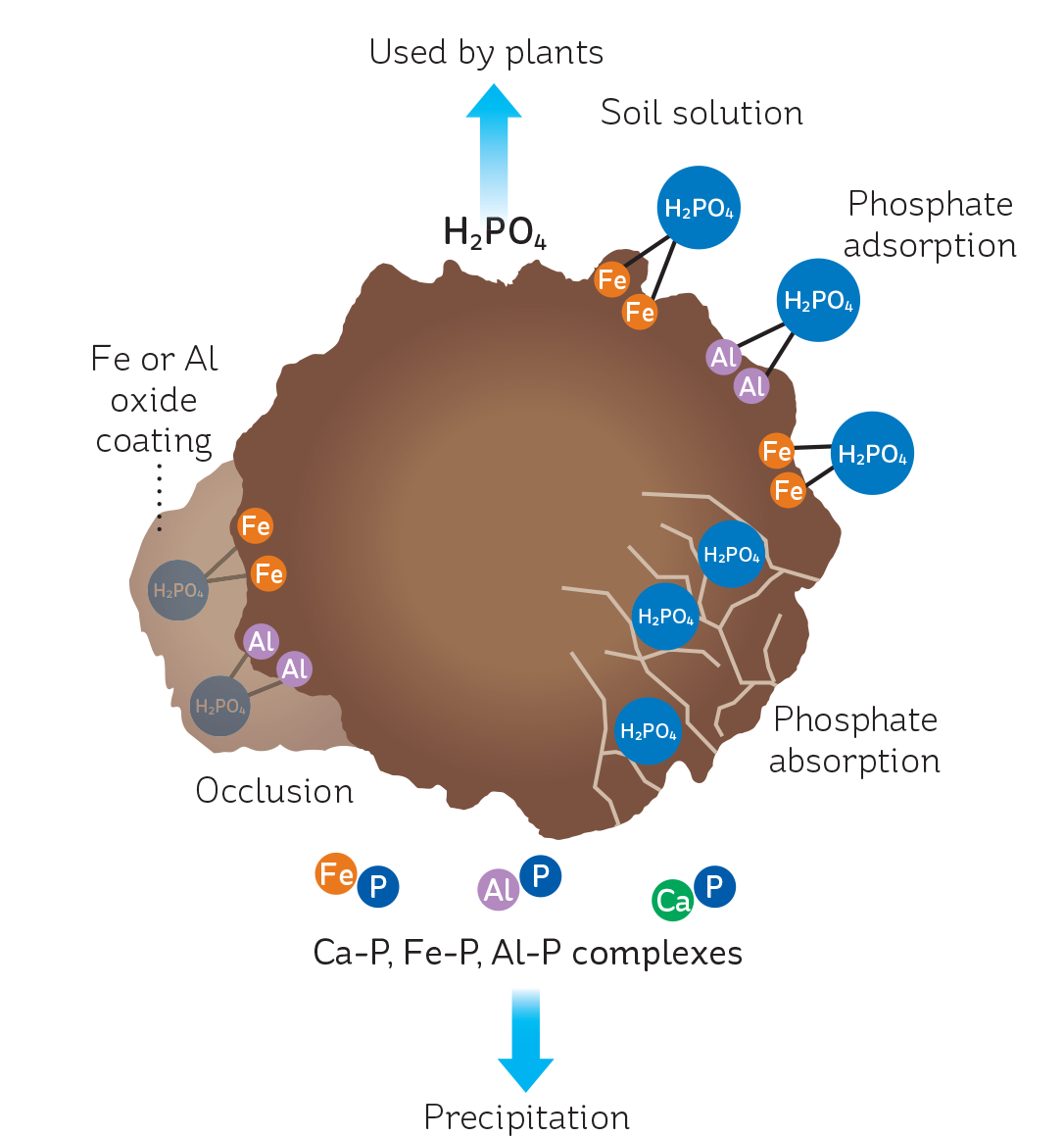
FIGURE 1: The fate of soluble phosphate added to soil. Adsorbed phosphate is readily released; absorbed and occluded phosphate less so. Insoluble complexes precipitated out of solution are unlikely to become available for plant uptake
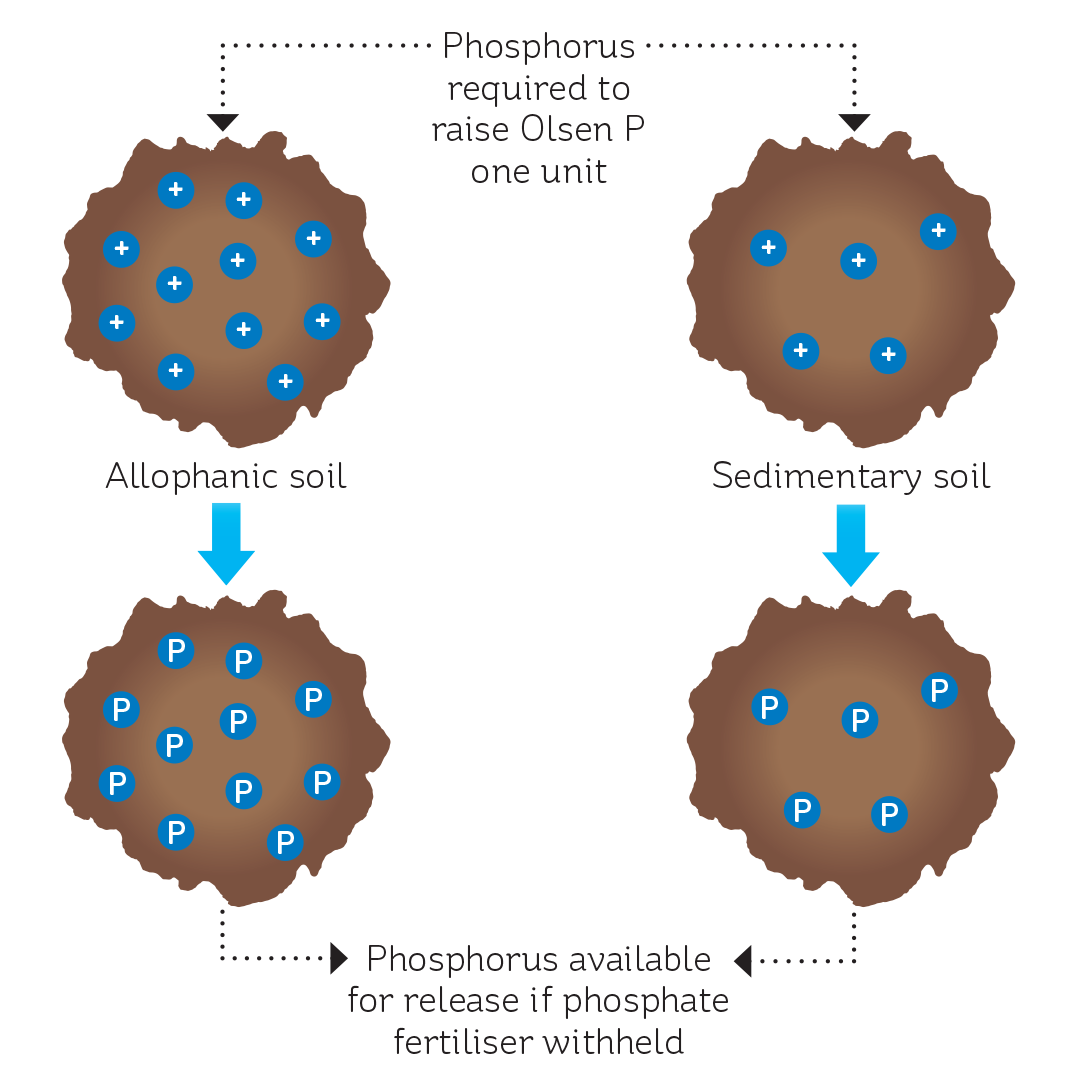
FIGURE 2: The effect of anion storage capacity on the capital phosphate fertiliser requirement and impact of withholding fertiliser